Vaccinations: Ingredients, Development, Myths, Regulations, and More
Article at a glance
Vaccines are composed of a variety of ingredients that each have a unique purpose. To learn more about the six main vaccine components and how they function, read this section.
For a vaccine to be licensed and widely approved, it must first go through five testing stages to ensure its safety and efficacy. To learn more about how a vaccine is developed, what each stage entails, and how a vaccine is monitored post-licensure, read this section.
Each virus is unique and complex, as are its vaccines. Due to each virus’s distinctive qualities, it’s necessary to administer each vaccine in a particular manner. To learn more about how vaccines are administered, read this section.
There are many myths surrounding vaccines and vaccine safety. To see how those myths debunked and the claims that created them, read this section.
In order to reduce the risk of epidemics and future outbreaks, several institutions require vaccines. To learn more about vaccination requirements on both federal and state levels, read this section.
Vaccinations are known to have a very long, robust history throughout time. The earliest recorded vaccine existed as early as 200 BCE. To learn more about the history of vaccines, read this section.
Vaccinations, otherwise known as vaccines, are preventative treatments given to humans to prevent them from various viruses and bacteria. Depending on the vaccine, they contain either a weakened or killed version of the same disease it’s working to prevent. As the weakened or killed germ enters the body, the immune system triggers antibodies to fight the virus. Our immune systems are built to store and remember how their antibodies fought off the virus. Therefore, if the real bacteria or virus were ever to enter the body again, the immune system already knows how to fight it, thus increasing our immunity to it. The World Health Organization explains that when a vaccine infiltrates our bodies, our immune system responds by acknowledging the bacteria, stimulating antibodies, remembering the bacteria, and how to fight it off. The Center for Disease Control (CDC) states that vaccines are a widely used medical tool because, “Unlike most medicines, which treat or cure diseases, vaccines prevent them.”
What are the Ingredients in a Vaccine?
According to the Center for Disease Control (CDC), vaccines contain a combination of preservatives, adjuvants, stabilizers, residual cell culture materials, residual inactivating ingredients, and residual antibiotics.
Preservatives
The purpose of preservatives in certain vaccines is to prevent the vaccine from contamination. The most common preservative used is thimerosal, and it’s primarily utilized in influenza (flu) vaccines. Thimerosal is a form of mercury, however, the CDC indicates that thimerosal is a different form of mercury than the kind that causes mercury poisoning. “Methyl mercury” is what causes mercury poisoning, while thimerosal is “ethylmercury.” Furthermore, vaccines use thimerosal or ethylmercury in small amounts, therefore it’s deemed safe for it’s processed differently in the body. To learn more about vaccine safety in regards to thimerosal, visit this source.
Adjuvants
Vaccines contain adjuvants to encourage the body to respond to the vaccine. A standard adjuvant used in vaccines is aluminum salts frequently found in drinking water, infant formula, antacids, aspirin, and antiperspirants.
Stabilizers
Stabilizers are used in vaccines to prolong its longevity after it has been manufactured. Common stabilizers include sugar or gelatin-like those included in jello.
Residual Cell Culture Materials
Residual cell culture materials, such as egg protein, increase vaccine growth to increase manufacturing capacity. Both influenza (flu) and yellow fever vaccines are developed in eggs, however, new flu vaccinations are available for those with egg allergies.
Residual Inactivating Ingredients
Residual inactivating ingredients are used to destroy toxins and viruses throughout the manufacturing process. Formaldehyde is the common residual inactivating ingredient used in vaccines because when diluted and processed in trace amounts, it poses no safety concerns.
Residual Antibiotics
Another way to limit a vaccine’s contamination during the manufacturing process is to use residual antibiotics as an ingredient. Neomycin is the common residual antibiotics used as it is least likely to cause an allergic reaction across the population.
Note: To learn more about vaccine ingredients and their roles in vaccine development, visit this source.
What’s the Difference Between Vaccinations and Immunizations?
Immunization is the process at large of how a person eventually becomes immune from a virus. The term “immunization” stems from the word “immune” or “immunity,” meaning that a person or being is unable to be harmed by an infectious disease. This immunization process consists of receiving one or a series of vaccines to boost immunity from a particular virus. Often the terms “vaccination” and “immunization” are used interchangeably. However, a vaccination or vaccine is merely a stepping stone within the immunization process.
Breakdown
Immunization
The process of becoming immune by receiving one or more vaccinations for a particular virus.
Vaccination
A step in the immunization process. Depending on the virus and the vaccine, the vaccination is administered one or more times with an end goal of the patient reaching immunity.
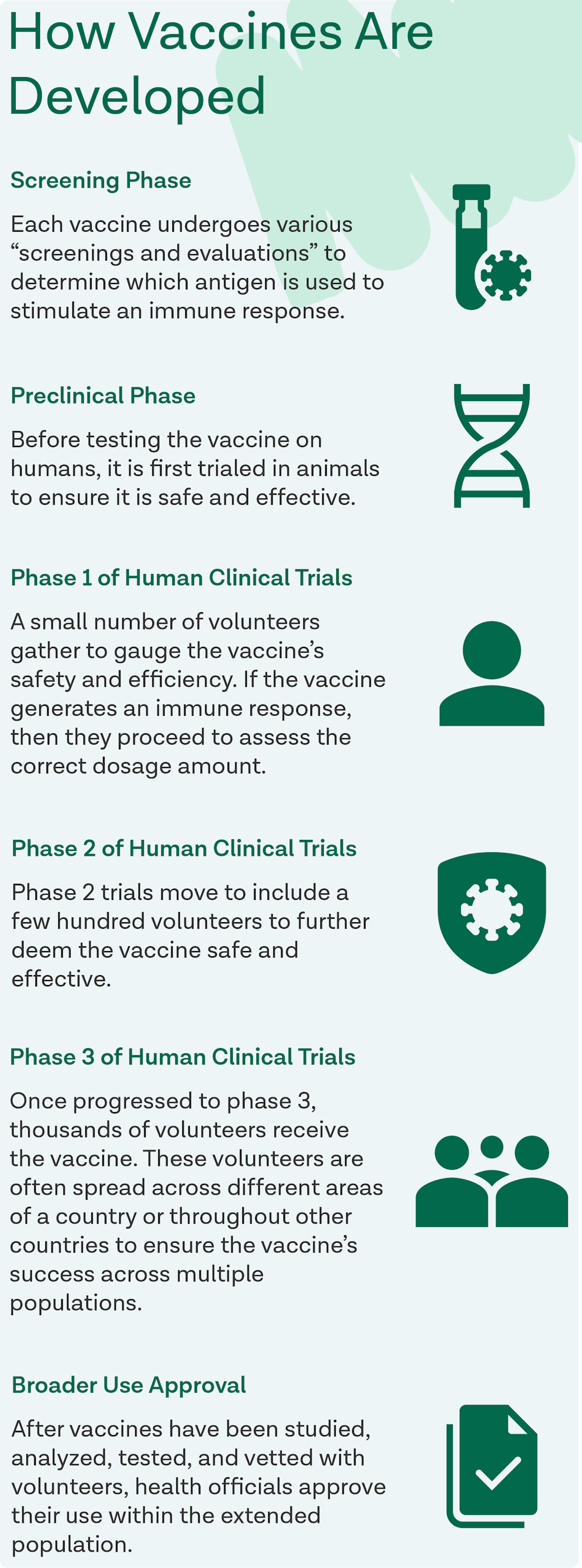
How Are Vaccines Developed?
Every vaccine developed is first tested through a series of phases to ensure its safety and efficacy. The World Health Organization (WHO) categorizes the stages as the screening phase, preclinical phase, phase 1 of human clinical trials, phase two of human clinical trials, phase 3 of human clinical trials, and lastly, broader use approval.
Vaccine Development Phases
Screening Phase
Each vaccine undergoes various “screenings and evaluations” to determine which antigen is used to stimulate an immune response.
Preclinical Phase
Before testing the vaccine on humans, it is first trialed in animals to ensure it is safe and effective.
Phase 1 of Human Clinical Trials
A small number of volunteers gather to gauge the vaccine’s safety and efficiency. If the vaccine generates an immune response, then they proceed to assess the correct dosage amount.
Phase 2 of Human Clinical Trials
Phase 2 trials move to include a few hundred volunteers to further deem the vaccine safe and effective. These volunteers stem from the young, healthy population to include participants whose characteristics (age, sex, etc.) align with those in the vaccine’s designated population. Participants undergo multiple trials in this phase to thoroughly evaluate each person’s response and experiment with various forms of the vaccine.
Phase 3 of Human Clinical Trials
Once progressed to phase 3, thousands of volunteers receive the vaccine. These volunteers are often spread across different areas of a country or throughout other countries to ensure the vaccine’s success across multiple populations. In both phase 2 and phase 3, some volunteers blindly receive a placebo vaccine, so scientists have unaffected volunteers to compare results to. Furthermore, to eliminate all bias, scientists are also shaded from knowing which volunteers received the vaccine and which received the placebo.
Broader Use Approval
After vaccines have been studied, analyzed, tested, and vetted with volunteers, health officials approve their use within the extended population. However, the WHO notes that the supervision of vaccine efficacy does not end there. They state that “once a vaccine is in use, it must be continuously monitored to make sure it continues to be safe.” Therefore, a system known as the Vaccine Adverse Event Reporting System (VAERS) is in place to conduct post-approval vaccine tracking. VAERS receives and studies reports of various side effects seemingly induced by a vaccine even after it is licensed. To learn more about VAERS and tracking post-licensed vaccines, visit this source.
Vaccine Safety Before & After Licensing
When a vaccine is produced, the main priority for all participating companies and agencies is safety. Due to this, there are many procedures and standards set in place to ensure that the vaccine is safe throughout the manufacturing process, the trial process, and beyond.
Before Licensing
Prior to a vaccine ever being administered or tested in humans, it is tested in laboratories. Sometimes the laboratory testing phase can take years but once the company producing the vaccine and the Food and Drug Administration (FDA) feel confident the vaccine is safe and effective, they progress it to clinical trials. Clinical trials advance in phases as shown above; starting with phase one and ending with phase three. Once the phases are complete and the vaccine is approved, testing continues to ensure the vaccine is “potent, pure, and sterile.” Potency is checked to guarantee the vaccine’s effectiveness; purity is checked to make sure certain ingredients that were used during production are entirely removed; and sterility is checked to certify that the final vaccine includes no outside germs. Throughout these final tests, the FDA continues to work closely with the company so all vaccine standards are met for both quality and safety. If all tests are complete and standards are reached, then the vaccine is finally able to be licensed and approved for societal use.
After Licensing
Once a vaccine is licensed and made available to the general public, the FDA, CDC, and other federal agencies work closely to continue monitoring the vaccine’s safety and efficacy. There are many systems in place that oversee and track all vaccines that are in operation throughout the United States. The top four systems include the Vaccine Adverse Events Reporting System (VAERS), the Vaccine Safety Datalink (VSD), the Post-licensure Rapid Immunization Safety Monitoring System (PRISM), and the Clinical Immunization Safety Assessment Project (CISA). Some federal agencies and offices also continue to research and test vaccines post-licensure such as the Department of Defense (DoD), the Department of Veteran Affairs (VA), the National Institutes of Health (NIH) and the Office of Infectious Disease and HIV/AIDS Policy (OIDP).
Types of Post-Licensure Vaccine Monitoring Systems:
Vaccine Adverse Events Reporting System (VAERS): VAERS is managed by the FDA and CDC to pinpoint potential vaccine safety issues. Patients, health care professionals, vaccine companies, etc. can all report adverse vaccine side effects to VAERS that the system then tracks and links to other cases to track if there’s an issue.
Vaccine Safety Datalink (VSD): VSD was made in collaboration with the CDC and many health care organizations nationwide as it processes medical records to track and research vaccine safety.
Post-licensure Rapid Immunization Safety Monitoring System (PRISM): The FDA put PRISM into play as a part of the Sentinel Initiative. This initiative focuses mainly on vaccine safety with PRISM observing the database of health insurance claims to spot if certain licensed vaccines are having issues.
Clinical Immunization Safety Assessment Project (CISA): CISA was made in collaboration with the CDC and vaccine safety experts from many nationwide medical research centers. The main goal of CISA is to carry out clinical vaccine safety research and occasionally evaluate complicated cases of patients experiencing odd side effects.
Note: To learn more about vaccine development and see it broken down in an infographic, visit this source.
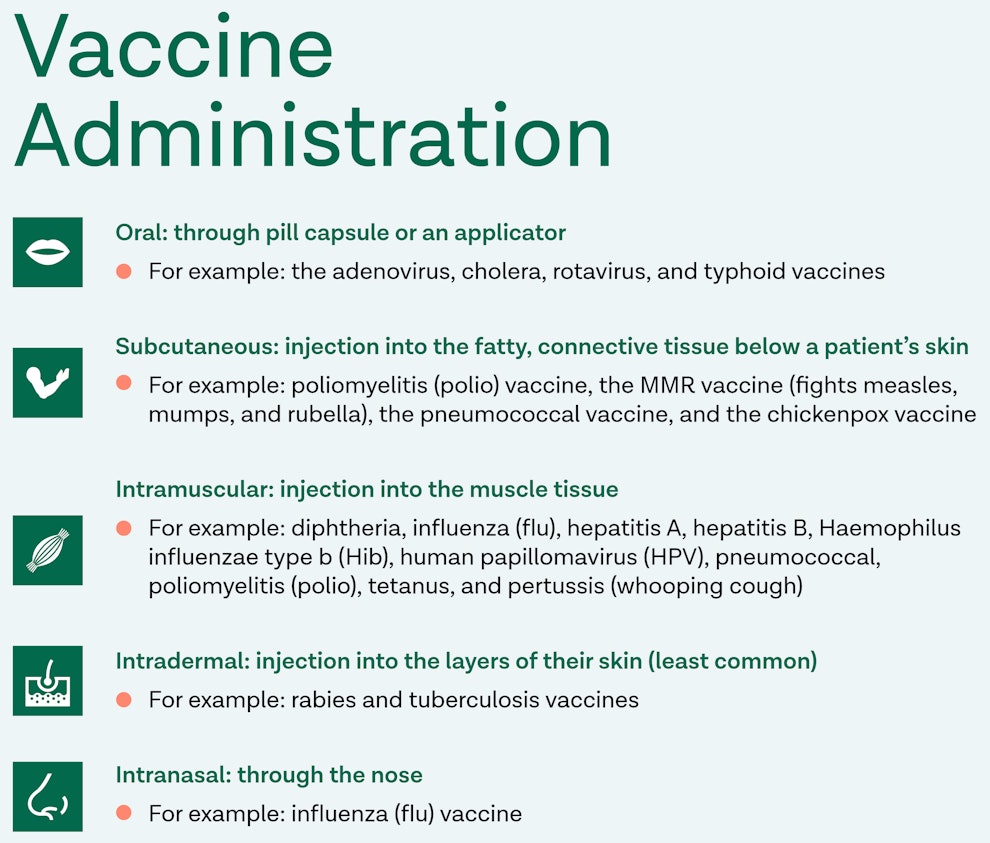
How are the Vaccines Administered?
There are five different methods for administering vaccines, depending on the vaccine and who is receiving it. The CDC lists the five vaccine routes as oral, subcutaneous, intramuscular, intradermal, and intranasal.
Oral
An oral vaccine enters a patient’s body through their mouth, either through a pill-like capsule or an applicator. There are currently only four common vaccines administered orally in the United States: the adenovirus, cholera, rotavirus, and typhoid vaccines. Rotavirus is a disease that primarily affects infants and children therefore, the rotavirus vaccine is recommended for children before the age of one. The vaccines for adenovirus, cholera, and typhoid are considered “non-routine vaccines” as these diseases are practically eradicated in the United States. However, they are made readily available for those traveling outside of the country where these diseases may cause concern.
Subcutaneous
Subcutaneous vaccines are administered through an injection into the fatty, connective tissue below a patient’s skin. Common subcutaneous vaccines include poliomyelitis (polio) vaccine, the MMR vaccine (fights measles, mumps, and rubella), the pneumococcal vaccine, and the chickenpox vaccine. Often, laboratories combine the MMR and Varicella vaccines into a joint vaccine known as the MMRV vaccine. It’s important to note that polio and pneumococcal vaccines are administered either subcutaneously or intramuscularly.
Intramuscular
A patient receives an intramuscular vaccine by injection into their muscle tissue. This form of administration is the most common with vaccines as there are ten different diseases fought with intramuscular vaccines. The ten diseases include diphtheria, influenza (flu), hepatitis A, hepatitis B, Haemophilus influenzae type b (Hib), human papillomavirus (HPV), pneumococcal, poliomyelitis (polio), tetanus, and pertussis (whooping cough). It’s common to vaccinate a patient for these diseases through a combination vaccine. For example, diphtheria, tetanus, and pertussis are often combined into one vaccine, as are the hepatitis A and B vaccines. It’s important to note that polio and pneumococcal vaccines can be administered either subcutaneously or intramuscularly.
Intradermal
A patient receives an intradermal vaccine through an injection in the layers of their skin. The intradermal form of administration is the least common among the five routes. The most common vaccine we see linked to this route is the flu vaccine. However, non-routine vaccines such as rabies and tuberculosis vaccines are known to be injected intradermally as well.
Intranasal
A patient receives an intranasal vaccine through their nose. This form of administration is also less common, however, we frequently see it used with influenza (flu) vaccine.
Vaccination Recommendations by Age Group
Recommendations from Birth to Age 6
The majority of vaccines are received when patients are recently born or are still young children. Recommended vaccines for those between birth and age six are the chickenpox, diphtheria, Hib, hepatitis A, hepatitis B, influenza, measles, mumps, rubella, pertussis, polio, pneumococcal, rotavirus, and tetanus vaccines. To learn more about when a child should receive these vaccines and how many doses are required, visit this source.
Recommendations for Those 7–18 Years of Age
The majority of recommended vaccines are given to children between birth and their first six years of life. Therefore, during their adolescent years, there are few shots they need to receive. A large portion of the vaccines adolescents receive are usually booster shots for specific viruses. The most common vaccines received during the years of 7 to 18 are the flu, meningococcal, and HPV vaccines alongside a Tdap (tetanus, diphtheria, and pertussis) booster shot. To learn more about the recommended vaccine and booster shots for those aged 7 to 18, visit this source.
Note: The meningococcal vaccination is often recommended for college or university students. To learn more about meningitis and when to receive the vaccine, visit this source.
Recommendations for Those 19–50 Years of Age
Most vaccines are typically recommended and received by those 18 years or younger. Therefore, adults 19 and older usually only need to get a Td (tetanus and diphtheria) booster every ten years, a flu vaccine annually, and a Tdap booster each time a woman is pregnant (preferably during the 27–36 week timeframe).
Recommendations for Those 50 Years or Older
Alongside the recommendation to receive a yearly flu vaccine and a Td booster every decade, those 50 years and older are encouraged to get a zoster vaccine. Additionally, those 65 years and older are encouraged to receive a pneumococcal vaccine.
Zoster Vaccine and Shingles:
Zoster vaccines work to prevent a common disease known as shingles. Shingles is a painful rash of blisters and scabs that commonly develops on the torso of older adults who have had chickenpox. While the rash normally fades within a month, many patients experience a long-lasting pain called postherpetic neuralgia (PHN) that can last for years after the rash heals.
There used to be two vaccines available for those over 50: Zostavax and Shingrix. However, Zostavax was discontinued in the United States as of November 18th, 2020. Shingrix, the preferred shingles vaccine, is a recombinant zoster vaccine that is given in two doses. Patients receiving the Shingrix vaccine get a shot in their upper arm. Two to six months following their first shot, they will receive another one to complete the vaccine that has over a 90% effective rate. The CDC encourages that all patients get vaccinated for shingles even if they’ve already received the Zostavax vaccine, experienced shingles in the past, or feel unsure about whether or not they’ve had the chickenpox.
To learn more about shingles and the Shingrix vaccine, visit this source.
Pneumococcal Vaccines:
There are two types of pneumococcal vaccines a patient is recommended to receive throughout their lifetime. One is labeled the PCV13 vaccine, and one is labeled the PPSV23 vaccine. The PCV13 vaccine is typically given to infants before they turn two years old. As a baby, this vaccine protects them from 13 severe strains of pneumococcal bacteria while they are young and vulnerable. However, as a patient gets older and reaches 65+, they grow susceptible to this invasive bacteria again, prompting them to receive the PPSV23 vaccine. The PPSV23 vaccine protects those 65+ from 23 types of pneumococcal bacteria that can lead to aggressive pneumonia, meningitis, and bacteremia infections. To learn more about pneumococcal vaccines, visit this source.
Common Vaccine Side Effects
It’s common for patients to experience minor side effects after being vaccinated, such as aches near the injection area, mild fever, chills, fatigue, and headaches. Severe side effects are infrequent, with only around 1 to 2 people affected per 1 million vaccinations given. If side effects continue or grow more severe after getting vaccinated, a patient should seek medical attention from their doctor. Each vaccine is different and connects to unique side effects. To learn more about the side effects associated with each vaccine, visit this source.

Reasons to Vaccinate & Not to Vaccinate
When evaluating whether or not to be vaccinated, it’s important to recognize that the pros for vaccinating outweigh the cons unless you are an individual that’s at higher risk of severe side effects.
Reasons to Get Vaccinated
Throughout the years, strides have been made towards eradicating certain diseases through vaccination increases. For example, severe infectious diseases such as smallpox have finally been expunged from society due to the world having more expansive access to vaccines. As stated before, vaccines are considered preventative medicine as their primary goals are to protect the vaccinated patient from a particular virus and work toward a world where this virus, and thus, vaccine, no longer exist. The CDC urges that if we saw a decline in vaccinations, viruses that we rarely hear of today would make a striking comeback. Not to mention, since vaccinated individuals are boosting their immunity to certain diseases, they are also preventing the spread of this disease to others in their circle. Therefore, vaccinations help to boost a patient’s own health and safety while protecting the health of their loved ones as well. To learn more about reasons to get vaccinated, visit this source.
Who Should Not Get Vaccinated
Due to certain factors like age and health conditions, it is recommended that some patients should not get vaccinated. With every vaccine, there are groups with contraindications or precautions. Contraindications mean conditions that indicate a patient is vulnerable to serious side effects or reactions to a vaccine. Meanwhile, precautions mean minor or acute illnesses that suggest a patient is more vulnerable to adverse side effects, and the vaccine’s efficacy may be compromised. While contraindications are viewed as more serious indicators than precautions, in most cases, both factors are temporary. To learn more about contraindications, precautions, and who should avoid being vaccinated, visit this source or this source.
Debunked Vaccine Myths
There are several myths surrounding vaccines that continue to cause patients confusion and hesitancy. However, the majority of these myths are grounded in illegitimate claims.
Myth #1: Vaccines cause the diseases or viruses they are trying to prevent.
Even though vaccines may occasionally stimulate mild side effects that allude to the disease/virus they are preventing, vaccines actually do not contain active viruses. This means that it’s nearly impossible for vaccines to infect you with the disease/virus they are preventing. The only case recorded where a vaccine was proven to cause disease was the Oral Polio Vaccine (OPV) which is now not in use in the U.S.
Myth #2: There is no need to get vaccinated because infection rates in the U.S. are minimal.
This myth stems from the fact that “herd immunity” does grant the unimmunized minority protection. However, a key part of herd immunity is that the majority of citizens need to be vaccinated in order for it to stay afloat and protect those, such as infants, pregnant women, elderly, and those with weakened immune systems, who cannot receive vaccines. If more people choose to not vaccinate, opportunities are opened for viruses and bacteria to spread and take a toll on our unimmunized population. Not to mention, with the factor of international travel at play citizens of other countries may be living in situations where certain diseases that are rare here, are considered a threat there.
Myth #3: Vaccines contain harmful toxins.
It is true that vaccines contain small amounts of formaldehyde, mercury, and aluminum. While first reading these ingredient names may signal red flags, they are safely included in vaccines in trace amounts that assist the vaccine to function properly. For example, formaldehyde helps to expunge outside toxins and viruses during the manufacturing process. Not to mention, it is produced at higher amounts in our natural bodies than any amount a patient is exposed to through a vaccine. In addition to this, the minute amount of mercury contained in a vaccine is through the ingredient thimerosal. However, thimerosal is a type of mercury that will not cause mercury poisoning because it is ethylmercury meanwhile, methyl mercury is the type that incites mercury poisoning. Furthermore, thimerosal was eliminated altogether from pediatric vaccines in 1999. Lastly, the aluminum included in vaccines are aluminum salts that are also commonly found in drinking water, infant formula, aspirin, etc. Vaccines undergo strenuous tests prior to being vetted for public usage therefore, the ingredients that may appear as red flags have been thoroughly cleared and safely approved.
Myth #4: Vaccines are linked to causing Autism.
This myth came about when Andrew Wakefield, a British surgeon, published a study in the prestigious The Lancet medical journal that the MMR vaccine was increasing autism in children. After discovering that Wakefield had financial incentives for publishing this piece and that there were several procedural errors and ethical violations, the paper was discredited and retracted from The Lancet and Wakefield proceeded to lose his medical license. Medical researchers and institutions went on to study Wakefield hypothesis to discover if there were any grounds to it at all but no link has ever been found between vaccines and autism.
Myth #5: Vaccine schedules are highly aggressive and should be more evenly spaced out.
The immunization schedules determined based on age and gender have been backed by decades of medical research that substantiate when a vaccine’s optimal time window is. Children are considered the most vulnerable population for their immune systems are not fully developed. This is why during their first six years of life they receive multiple vaccines during specific years to ensure they are accurately being protected from ruthless diseases/viruses.
Myth #6: It’s dangerous to vaccinate children so early in life.
As stated before, children are the most vulnerable population because their immune systems are not fully formed. Not vaccinating them when they’re young puts them at risk of having life-threatening diseases such as whooping cough, measles, and diphtheria. Some parents bolster this myth because of their belief that children are receiving too many antigens. However, the amount of antigens has steadily increased since the 1980s due to vaccines becoming more effective. In the 1980s, the immunization schedule included 3,000 antigens, meanwhile today’s schedule only includes 35 with each vaccine containing anywhere from 1 to 13.
Myth #7: Natural immunity is better than vaccine immunity.
While natural immunity can result in stronger immunity to a disease, more dangers lie in this approach than benefits. For example, if a patient wanted to gain immunity to the measles and decided to do it naturally by exposing themself to the disease, then they would be up against a 1 in 500 chance of dying. Meanwhile, if they received the MMR vaccine their chance of having a severe allergic reaction is one in a million. Deciding to naturally gain immunity to certain diseases that have taken lives for years vice gaining immunity through a vaccine, is not advised.
Myth #8: Better hygiene is responsible for decreased infection rates, not vaccines.
Ignoring the role hygiene and sanitation play in decreasing infection rates would be ignorant, however, decades of research have proved vaccines’ efficacy in minimizing infection rates. For example, the first measles vaccine was introduced in 1963 when the rates of infection had been stable at about 400,000 cases per year. With hygienic and sanitation habits going generally unchanged over the following decade, by 1970 cases were down to about 25,000 cases per year. This same trend occurred with Hib disease where the vaccine was introduced in 1990 with 20,000 cases per year and by 1993 there were only about 1,500 per year.
Where and How Vaccines are Required
Federal Guidelines
As of now, vaccines are not federally mandated and are instead implemented on a state-by-state basis for particular institutions. The Congressional Research Service notes that the federal government plays a “limited role” in the country’s vaccine regulation. Congress has afforded federal health authorities “broad, flexible powers” through the Public Health Service Act (PHSA) to enforce if a disease is widespread and infectious. Despite this power, the federal government currently only requires that two populations get vaccinated: military personnel and immigrants seeking permanent residence.
State-by-State Guidelines
Even though a federal mandate is not in effect, states impose vaccine regulations for various institutions to lower the rates of vaccine-preventable diseases (VPDs). Vaccination laws are in place for children in daycare and public/private school settings, college/university students, healthcare workers, and patients residing in certain facilities. However, while each state does require vaccines by law, each state also permits exemptions for religious or medical reasons. To learn more about the kinds of exemptions allowed and how they are administered in your state, visit this source.
Brief History of Vaccinations
Vaccines are claimed to be brought into existence as early as 200 BCE. The earliest recorded instance of a vaccination taking place was in the year 1,000 when a series of images emerged displaying a man covered in boils with a swab next to him. These drawings are alluding to the smallpox vaccination discovery, which ancient records corroborate. Overtime, vaccinations have grown and flourished, leading to the prevention of vicious diseases that used to plague our nation and world. For example, diseases such as measles, rubella, whooping cough, polio, etc. now have vaccines that protect patients from being infected. Some diseases have even been eradicated in the United States, including smallpox, polio, and the measles. Known as one of the greatest inventions of all time, vaccines are backed by centuries of development and decades of research. Gradually, they have become a key tool in preventive medicine, leading us to successfully eliminate or vastly diminish many diseases that used to harm us.
Note: Vaccines have a long, detailed history throughout the world. To learn more details about the history of vaccine development and disease discovery, visit this source.
Sources
- https://www.who.int/news-room/q‑a-detail/vaccines-and-immunization-what-is-vaccination
- https://www.historyofvaccines.org/timeline/all
- https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm
- https://www.cdc.gov/vaccines/vac-gen/side-effects.htm
- https://www.cdc.gov/vaccines/vpd/should-not-vacc.html
- https://www.cdc.gov/vaccines/hcp/admin/administer-vaccines.html
- https://www.who.int/news-room/feature-stories/detail/how-are-vaccines-developed
- https://www.cdc.gov/vaccines/vpd/vaccines-age.html
- https://fas.org/sgp/crs/misc/LSB10300.pdf
- https://www.cdc.gov/vaccines/imz-managers/laws/state-reqs.html
- https://www.cdc.gov/phlp/publications/topic/vaccinationlaws.html
- https://www.cdc.gov/phlp/publications/topic/vaccinations.html
- https://vaers.hhs.gov/index.html
- https://www.cdc.gov/vaccines/basics/test-approve.html
- https://www.phe.gov/Preparedness/planning/authority/Pages/default.aspx
- https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- https://www.cdc.gov/flu/prevent/egg-allergies.htm
- https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/administration.html
- https://www.cdc.gov/vaccines/vpd/vaccines-diseases.html
- https://www.who.int/bulletin/volumes/89/3/10–079426/en/
- https://www.vaccines.gov/basics/safety/side_effects
- https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.html
- https://www.cdc.gov/vaccines/vpd/vaccines-age.html
- https://www.cdc.gov/vaccines/vpd/shingles/index.html
- https://www.vaccines.gov/get-vaccinated/for_parents/five_reasons
- https://www.vaccines.gov/basics/safety
- https://www.publichealth.org/public-awareness/prenatal-care/vaccine-myths-debunked/
- https://www.rush.edu/news/7‑myths-about-vaccines
- https://www.cdc.gov/vaccines/parents/infographics/journey-of-child-vaccine.html
- https://www.cdc.gov/vaccines/vpd/shingles/public/shingrix/index.html
- https://www.cdc.gov/shingles/vaccination.html
Become a patient
Experience the Oak Street Health difference, and see what it’s like to be treated by a care team who are experts at caring for older adults.