Article at a glance
There are many benefits to receiving the COVID-19 vaccine such as personal health and the protection of those around you, as these vaccines prevent severe outcomes from COVID-19 infection such as serious illness, death, and more.
As of October 2023, there are now three authorized vaccines available including vaccine booster doses.
The Center For Disease Control And Prevention (CDC) currently recommends anyone ages 6 months and older get vaccinated against COVID-19.
Those who should not get a COVID-19 vaccine include people who have experienced severe allergic reactions to any mRNA vaccine.
After the World Health Organization declared the COVID-19 outbreak an official pandemic in 2020, the race to produce a safe and effective vaccine was on. While vaccines normally take a minimum of six years to be developed, studied, and manufactured, the Moderna and Pfizer coronavirus vaccines were readily produced within a year. Due to modern technological advances and past studies linked to gaining immunity over COVID-19, the vaccines were able to be produced at rapid speeds.
Today, there are now three updated vaccines that are approved by the Food and Drug Administration (FDA) for COVID-19. This includes an updated Novavax vaccine, Moderna vaccine, and Pfizer vaccine.
This article will explain everything you need to know about these COVID-19 vaccinations including vaccine types, current CDC recommendations, information on updated COVID-19 vaccines as of 2023, and more.

Vaccine Types
There are currently three vaccines available: Moderna, Pfizer, and Novavax. Each of these vaccines has an updated version for 2023–2024. (Note: The Johnson & Johnson vaccine is no longer available for use in the United States.)
Pfizer-BioNTech Vaccine
During clinical trials, the Pfizer vaccine was proven 95% effective against COVID-19. It’s an intramuscular vaccine given in a patient’s upper arm and patients receive the vaccine in two doses.
After the first vaccine dose, the patient should get their second vaccine 21 days later. After the initial two doses, patients may have received an initial (monovalent) booster dose two months after their last dose of their primary series of vaccinations.
Note: Patients must receive the same vaccine (i.e either Pfizer or Moderna) for their initial booster dose. Learn more about vaccine booster doses at this resource.
Who Shouldn’t Get the Pfizer Vaccine:
Anyone who has experienced a mild, severe, or immediate allergic reaction to any mRNA vaccine (including the first dose of Pfizer’s COVID-19 vaccine), polyethylene glycol (PEG), and/or polysorbate, should not get the Pfizer COVID-19 vaccine.
Note: An immediate or severe reaction means experiencing hives, swelling, or wheezing within four hours of receiving an mRNA vaccine.
Common Side Effects:
During clinical trials, most people got mild to moderate side effects while a few had severe side effects. These side effects are prone to start within one to two days after getting vaccinated and should only last a few days at maximum. The side effects related to the Pfizer COVID-19 vaccine include:
- In Arm:
Minor swelling
Pain
Irritation/redness
- Throughout Body (common after the second dose):
Chills
Mild fever
Tiredness
Headache
Note: If severe side effects occur or moderate side effects persist for longer than a few days, be sure to contact your doctor.
Moderna Vaccine
During clinical trial testing, the Moderna vaccine was proven 94.1% effective against the coronavirus. The vaccine is an intramuscular shot given in the patient’s upper arm and patients receive the vaccine in two doses that are given 28 days apart.
Who Shouldn’t Get the Moderna Vaccine:
Anyone who has experienced a mild, severe, or immediate reaction to any mRNA vaccine (including the first dose of Moderna’s COVID-19 vaccine), polyethylene glycol (PEG), and/or polysorbate, should not get the Moderna COVID-19 vaccine.
Note: An immediate or severe allergic reaction means experiencing hives, swelling, or wheezing within four hours of receiving an mRNA vaccine.
Common Side Effects:
During clinical trials, most people got mild to moderate side effects while a few had severe side effects. These side effects are prone to start within one to two days after getting vaccinated and should only last a few days at maximum. The side effects related to Moderna’s COVID-19 vaccine include:
- In Arm:
Minor swelling
Pain
Irritation/redness
- Throughout Body (common after the second dose):
Chills
Mild fever
Tiredness
Headache
Note: If severe side effects occur or moderate side effects persist for longer than a few days, be sure to contact your doctor.
Novavax Vaccine
The Novavax vaccine is the most recent vaccine approved by the FDA. People 12 and older are able to receive the Novavax vaccine. A Novavax booster can be given six months after the first dose. Americans 12 through 17 who received the Novavax primary series must get a Pfizer-BioNTech bivalent booster.
Who Shouldn’t Get the Novavax Vaccine:
The Novavax vaccine is not recommended for anyone under the age of 12 years. As well, anyone who has experienced a mild, severe, or immediate reaction to the ingredients found in the COVID vaccine should not get the Novavax vaccination.
Common Side Effects:
Side effects related to the Novavax COVID-19 vaccine include:
Fatigue
Malaise
Muscle pain
Headache
Joint pain
Nausea and vomiting
Fever
Chills
Note: If severe side effects occur or moderate side effects persist for longer than a few days, be sure to contact your doctor or another healthcare provider.
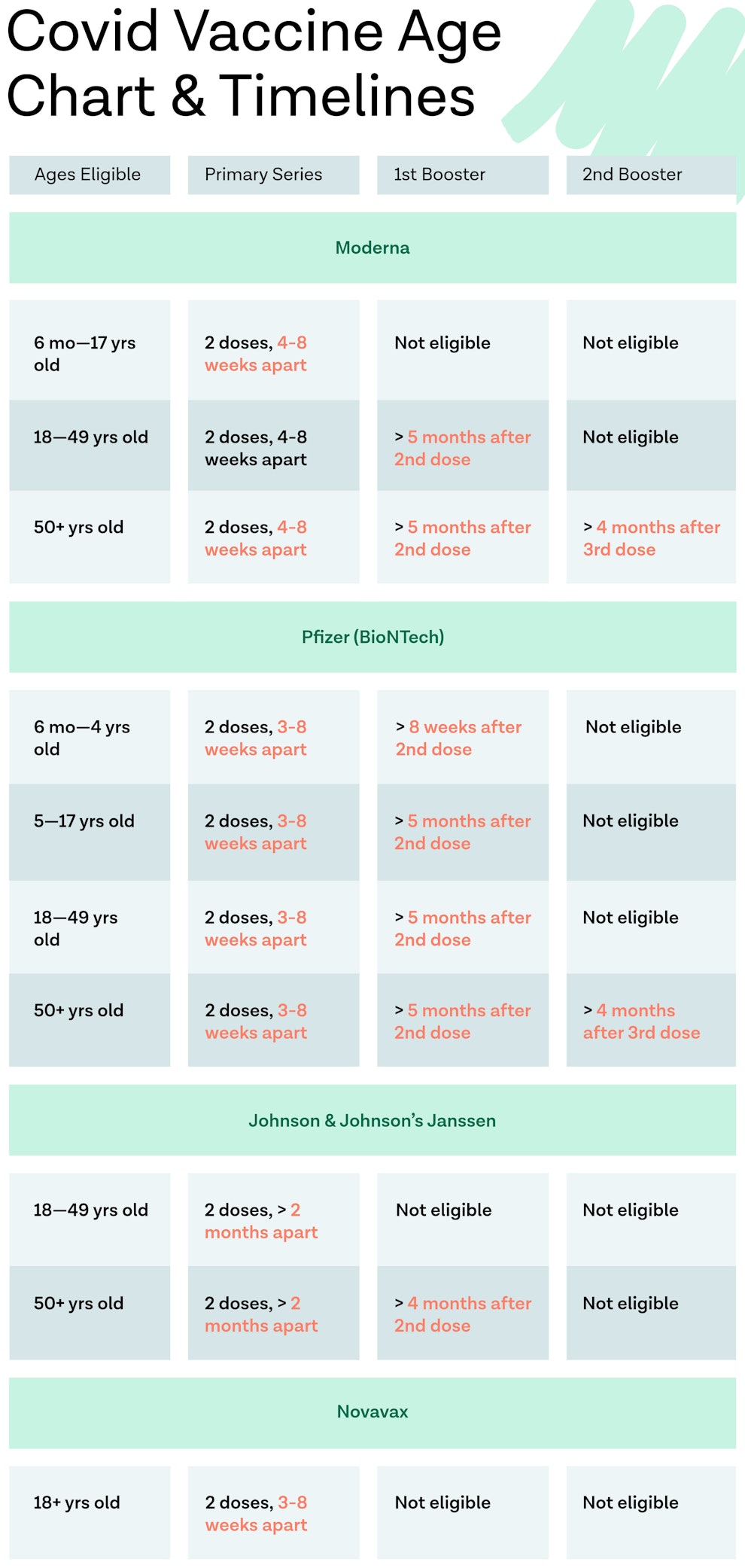
Updated COVID-19 Vaccine Information: 2023
As of October 2023, the FDA has approved and authorized for the emergency use of the 2023–2024 formula of updated COVID-19 vaccines from Pfzier, Moderna, and Novavax. This formulation includes a monovalent component that corresponds to the omicron variant XBB.1.5 of SARS-CoV‑2.
The CDC currently recommends everyone 5 years and older to receive one dose of an updated COVID vaccine. People 12 years and older who were not previously vaccinated should either get one updated Pfizer-BioNTech or one dose of the updated Moderna COVID-19 vaccine or two vaccine doses of the updated Novavax COVID-19 vaccine.
Immunocompromised individuals should get multiple doses administered (i.e. two to three doses) of the same brand of COVID vaccine. Additional doses may be available as well. Learn more about the COVID-19 vaccination schedule for those with a weakened immune system here.
Vaccinations and Those Over 55 Years of Age
Many questions have been raised surrounding both vaccines’ safety with those 55+ years old and those with comorbid conditions. However, clinical trials proved that COVID-19 vaccine side effects were actually less common and severe within these populations. One study notes that the vaccine is “better tolerated in older adults than younger adults” and that its efficacy is similar across all age groups.
Note: To read personal accounts from those 55 and older on receiving the COVID-19 vaccine, visit this source.
Benefits of Getting Vaccinated Against COVID-19
Getting vaccinated for COVID-19 has several benefits including preventing severe illness, hospitalization, and death. Learn more about the benefits of COVID-19 vaccination below.
Protecting Yourself from COVID-19
Clinical trials have determined that COVID-19 vaccines are highly effective at preventing a patient from contracting the coronavirus. The Pfizer vaccine was proven to be 95% effective against the coronavirus during clinical trials while the Moderna vaccine was proven to be 94.1% effective against the coronavirus during clinical trials. Furthermore, past vaccine research shows that if a patient does get infected, being vaccinated helps to protect the patient from severe disease.
Protecting Others from COVID-19
When a person is infected with the coronavirus, they’re considered very contagious. It’s possible for an infected person to spread the disease to their loved ones through respiratory droplets when they breathe, sneeze, cough, etc. In order for each individual to protect themselves and the ones they love from being infected, the CDC emphasizes getting vaccinated. While patients who have already contracted and healed from the coronavirus do have natural immunity, experts are unsure how long this immunity will last. Due to this uncertainty, the CDC is recommending that at the appropriate time, those who can, should be vaccinated.
Booster Doses
The CDC recommends for anyone ages six months or older. COVID-19 vaccine booster doses are recommended for those ages five years and older when eligible. Use the “Find Out When You Can Get Your Booster” tool on this page of CDC website to see when you’re eligible for your COVID-19 vaccine booster shot. Currently, all vaccine manufacturers have booster shots available.
COVID-19 Vaccine FAQs
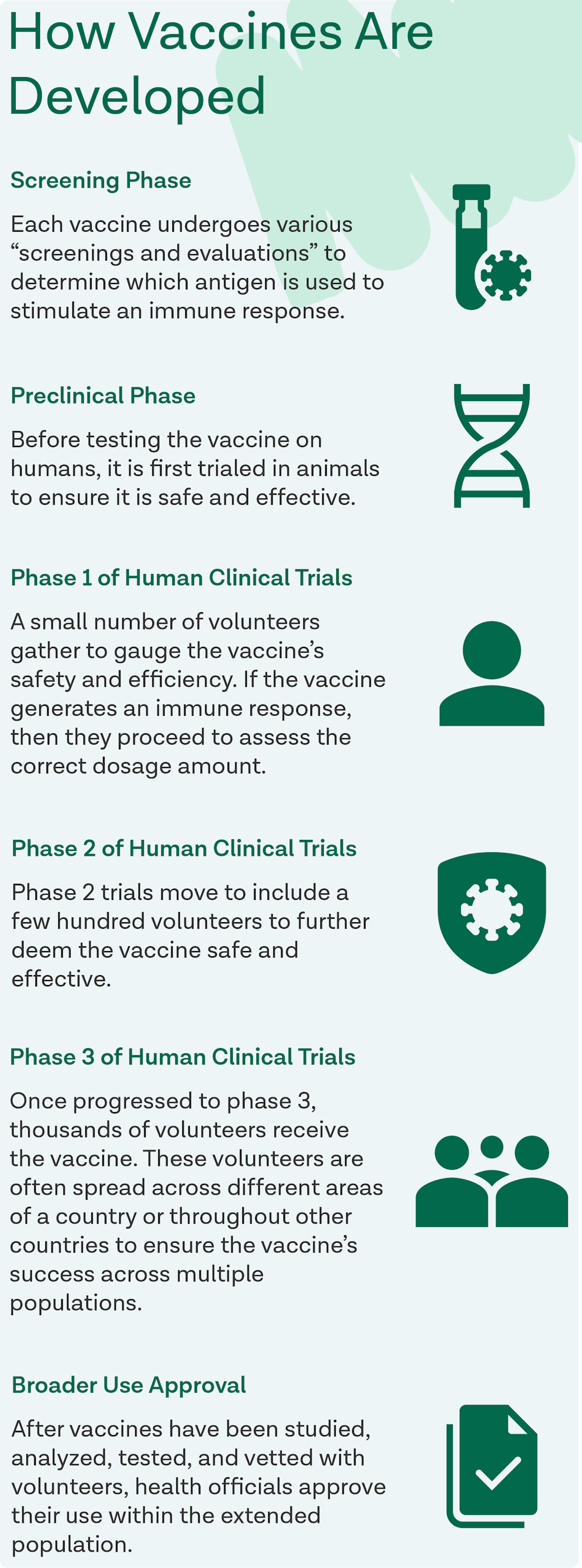
How were the vaccines developed so quickly?
How were the vaccines developed so quickly?
The COVID-19 vaccines were developed so rapidly due to a variety of factors:
Previous research demonstrated the role of the spike protein in coronavirus pathogenesis and displayed evidence that proved the importance of neutralizing that protein in order to reach immunity.
Nucleic acid technology has rapidly evolved, allowing vaccines to be created and manufactured much quicker than in years prior.
Development activities were able to be conducted in a parallel manner vice sequentially which hastened vaccine testing and manufacturing without putting participants at risk.
How many doses are considered to be full vaccination?
In order to be considered as fully vaccinated, one must have received the complete primary series of a vaccine and received the most recent booster vaccination recommended by the CDC.
Are COVID-19 vaccines safe?
Yes. In fact, hundreds of millions of Americans have received COVID-19 vaccines under the most intense safety monitoring in the United States. Learn more about the safety of vaccination for COVID-19 at this resource.
Sources
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Fabout-vaccines%2Fhow-they-work.html
- https://www.fda.gov/media/144414/download
- https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
- https://www.fda.gov/media/144638/download
- https://www.ecdc.europa.eu/en/covid-19/facts/questions-answers-basic-facts
- https://www.hackensackmeridianhealth.org/HealthU/2021/01/11/a‑simple-breakdown-of-the-ingredients-in-the-covid-vaccines/
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- https://www.nejm.org/doi/full/10.1056/NEJMe2025111
- https://www.aarp.org/health/conditions-treatments/info-2020/covid-vaccine-experience.html
- https://www.vaccines.gov/
- https://www.jhsph.edu/covid-19/articles/side-effects-and-covid-19-vaccines-what-to-expect.html
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32481–8/fulltext
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html#viral-vector
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/safety-of-vaccines.html
- https://www.cdc.gov/coronavirus/2019-nCoV/index.html
- https://www.fda.gov/media/146305/download
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html#protein-subunit
- https://www.who.int/news-room/feature-stories/detail/the-novavax-vaccine-against-covid-19-what-you-need-to-know
- https://www.fda.gov/media/159898/download
- https://www.ksby.com/news/coronavirus/pfizer-to-start-charging-for-covid-19-vaccines-when-government-funding-stops
- https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines-2023–2024
- https://www.cnn.com/2023/05/15/health/johnson-johnson-covid-vaccine-end/index.html
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
- https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#immunocompromised
Johnson & Johnson Vaccine
This infographic breaks down the Johnson & Johnson/Janssen COVID-19 vaccine.